SSRL Battery Plate Chemicals
Shree Sai Research Lab. deals with the manufacturing of battery plate electrolyte additives. The chemicals are SSRL's unique innovation and currently most trusted and reliable battery manufacturing companies are using them. We are the Global Players in supplying innovative battery plate chemicals to improve quality of lead acid batteries.Below is our product list :
Sodium Sulfate LR Grade Anhydrous Tablets | Download |
Sodium Sulfate LR Grade is a battery life saver which prevents battery by early premature failure It is used in electrolyte of free flooded conventional lead acid & VRLA batteries. By adding common ions (Sulfate Ions) to the acid electrolyte reaction, it will increase the sulfuric acid concentration and hard ions of lead Sulfate will soluble so fast, so that the specific gravity increases up to a level in all cells of lead acid batteries.
Product Image :

How Sodium Sulfate Tablets Work In Lead Acid Batteries?
Sodium Sulfate are highly water soluble salts and they dissociate in water, producing Sulfate ions. Concentration of these salts have been kept at such level that conductivity is high and this helps electrical current to pass through it.
The addition of sodium Sulfate provides an inventory (excess) of Sulfate ion that are available for more conductance. the growth of larger crystals, called hard Sulfate occurs by a dissolution precipitation process when the battery is in a discharged condition.
Lead Acid Battery Reactions : The reaction in lead acid battery is reversible as shown below :
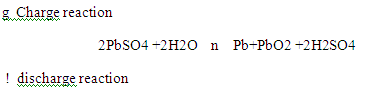
Sulfate ions are common in both charge and discharge reactions. The reaction is also known as double Sulfate theory. During discharge lead Sulfate builds up and becomes insulating material, thereby increasing internal resistance of the cell / battery. If another common conducting ion like dissociated sodium Sulfate accepts charge of the cell / battery. Whenever a solution of an ionic substance comes into contact with another ionic compound with a common ion, the solubility of the ionic substance decreases significantly. In case of Charging When Na2SO4 is added as additive in acid electrolyte, then the concentration of Sulfate ion increases, so that the solubility of PbSO4 decrease.
On the other hand in case of discharging : When Na2SO4 is added as additive to in an electrolyte medium Diluted Sulphuric acid then the concentration of Sulfate ion increases, so that the solubility of PbSO4 increase.
According to famous scientist, Lechatelier & his hypothesis that, when a chemical reactants are at equilibrium, the addition of more of one of the ions from another compound will shift the composition to the left, reducing the concentration of other ion and effectively reducing the solubility of the solid to maintain the equilibrium.
By Adding Sodium Sulfate Sulfate into lead Sulfate, it will increases the concentration of Sulfate ions in Lead Sulfate, So that Formation of Sulfuric acid will be more faster to attend the equilibrium.
By Adding Sodium Sulfate ions as electrolyte additive into lead acid batteries, the cell reaction becomes faster for the production of Lead Sulfate in case of discharging and sulfuric acid in case of charging.
Material Specification : Sodium Sulfate LR Grade Anhydrous - Na2SO4
| Test | Result |
| Appearance | White Colored Tablets |
| Assay | 99.0% Na2SO4 Min. |
| pH of a 10% solution | 7.2-8.2 at 270C |
| Solubility | 100 % Soluble in Water |
| Loss on ignition | 0.5% Max |
| Chloride (Cl) | 20ppm Max |
| Nitrogen compounds (as N) | 5 ppm Max |
| Phosphate (PO4) | 0.005% Max |
| Heavy metals (as Pb) | 5 ppm Max |
| Iron (Fe) | 20 ppm Max |
| Calcium (Ca) | 0.005% Max |
| Magnesium (Mg) | 0.005% Max |
| Potassium (K) | 0.005% Max |
Fast Cure Cum Binder (FCCB) | Download |
Fast Cure Cum Binder : SLI & VRLA Positive electrode Paste Additive (For Automotive Positive Plate)
The positive plates in an SLI (Starting Lighting Ignition) lead-acid battery are made from lead-alloy grids pasted with active material. The active material on the positive plates is mainly a mixture of lead oxide and sulphuric acid. In order to achieve a porous structure with a high surface area for the electro-chemical process, the pasted positive plates undergo a Paste preparation & curing process to transform the lead oxide and lead sulfate into basic lead sulfates – either Tribasic or Tetrabasic lead sulfate. There is however limitations due to the large size of the Tetra basic lead sulfate crystals. This can lead to a major disadvantage with Tetra basic lead sulfate namely a prolonged and higher electrical energy requirement for the formation of the positive plates and less surface area for the electro-chemical processes.
For Positive electrodes Fast Cure Cum Binder provides high energy density and high cycle lives suitable for in vehicles & other applications. It increases the conductivity flow from the positive active material to the current collector lead alloy. Fast Cure Cum Binder provides binding protective shell to paste while charge and discharge mechanism. The formation of a homogenous tetra-basic sulfate crystal structure in the positive plates of lead-acid batteries is well known to offer significant electro chemical improvements in battery performance in respect of improved cycling and battery life. A number of methods for obtaining the tetra-basic lead sulfate crystals by simply heating the pasted plates during curing to more than 80OC, for example during a short steam cure will generate a significant tetra-basic crystal structure although with a wide ranging crystal size distribution, sized of more than 50 microns being common. Therefore, figure 1 shows big sized tetra basic lead sulfate crystals formed after a steam cure step without any other additives.
Figure1 shows creating the tetra-basic structure is to control the crystal size distribution. Tetra-basic crystals, in comparison to tribasic crystals, are much harder to create. As the lead sulfate crystals develop there may be a limited number of developing crystal seed sites due to a higher activation energy compared to tri-basic and hence it is common to produce a relatively wide distribution across a plate with a number of very large crystals. These larger tetra-basic crystals lead to problems in the later formation of the plates as evidenced by an increased specific energy consumption and extended formation times when compared to tri-basic active materials.
By addition of Fast Cure Cum Binder a number of crystal seed sites are created, this leads to a large number of relatively even tetra-basic lead sulfate crystals. Trials carried out Fast Cure Cum Binder with approx. 4 to 5 micron particle size, showes some benefits with an improvement in the homogeneity of the crystal size distribution.
The main means of generating the crystals has in general been the heating of the pasted plates to more than 80OC in a steam cure process. By Use of Fast Cure Cum Binder, it has been found that only a relatively short treatment of 2 to 3 hours is required to fully develop the crystal structure. Figure 2 & 3 shows a tetra basic lead sulfate structure formed after a steam cure step using 1 % of Fast Cure Cum Binder powder with a particle size of approx. 5 micron as a seeding crystal. Pilot trials with Fast Cure Cum Binder showed benefits in thecuring and formation is SLI plate production related to process time reductions and energy savings. The Fast Cure Cum Binder was added as approx. 1%, of the lead oxide content in the batch. Some formulation, very often in thepaste preparation of the acid-oxide ratio has been reduced to minimize the possibility of temperature spikes in the paster. These temperature spikes can lead to uncontrolled tetra basic crystal formation. With Fast Cure Cum Binder can follow regular paste preparation method or can increase acid-oxide ratios from 7 or 8 liters 1.400 sp. gravity per 100kg lead oxide giving higher porosity values for the paste.
Curing and Crystal Size Bonding
There are two main ways of curing taking into account the different process steps and parameters available : the mixture of lead oxide and lead sulfate of the freshly manufactured positive plate is transformed into either Tribasic lead sulfate (PbSO *3PbO*H O) or Tetra basic 4 2 lead sulfate(PbSO *4PbO*H O). A Tetrabasic curing of 4 2 the plates is preferred versus a Tribasic curing because of the enhanced performance of the batteries. Batteries made from Tetra basic formed positive plates are known to last longer in all applications, compared to those made from Tribasic formed plates. The main disadvantage of the Conventional Steam Oven Tetrabasic curing process is the appearance of relatively large Tetrabasic lead sulfate crystals. The size/shape of the Tribasic as well as Tetrabasic lead sulfate crystals is controlled by temperature and humidity. Up to now it is has not been possible to control precisely the size of the final Tetra basic lead sulfate crystals.
Nucleation theory explains the differences : Tri basic lead sulfate nuclei become stable at small sizes at low temperatures -50°C. They are many in number and grow until the lead sulfate ions are exhausted. The result is a small sized Tri basic lead sulfate crystal structure.
Tetra basic lead sulfate nuclei became stable only at larger sizes. These sizes will be obtained only at higher temperatures, >60°C, (by use of steam). Relatively few nuclei are generated and grow until the lead sulfate ions are exhausted. Because of the relatively low number of nuclei they tend to be large, around 50µm and more influenced to some extent by the content of sulphuric acid in the paste preparation method. Even though the process time for curing to Tetra basic lead sulfate is shorter than for Tribasic lead sulfate the large crystals give rise to disadvantages with respect to formation and internal surface area.
The uncontrolled Tetra basic crystal growth leads to a reduction in the active surface of the plate. A high specific surface area is demanded for good battery performance with respect to high current discharge capability. The main feature of the Fast Cure Cum Binder is that size of the final Tetrabasic lead sulfate crystals can be controlled within the range from 3 to 15 µm by the paste preparation method, dependent on the amount of Fast Cure Cum Binder added to the lead oxide. Small crystals with a narrow distribution exhibit a larger specific surface in the plate thereby increasing the high current discharge performance of the plate with an advantageous Ah/kg ratio. Fast Cure Cum Binder allows the reduction of the curing time by use of steam and the small crystal size reduces the formation time. Consequently the use of FAST CURE CUM BINDER will reduce both the production costs and can lead to a significant reduction in working capital. Furthermore the improved cycling behavior of the batteries will improve the battery life.
It is a generally held belief that the reduction of the residual metallic lead is only achieved within such a curing step. It is easy to prove that the free lead reduction can place during the drying phase by ensuring that there is sufficient oxygen/air available. Nevertheless the appearance of large Tetrabasic lead sulfate crystals of 50 µm and above give problems during formation and reduce the specific surface area. It has been well tested that the addition of Fast Cure Cum Binder generates small and controlled sized Tetra basic lead sulfate crystals.
The Fast Cure Cum Binder additive is a very fine-sized and specially treated Tetra basic lead sulfate with Carboxymethylcellulose. Within the production process, an average grain size of 0.5 µm is obtained. The tendency of such small particles to re-agglomerate is avoided by addition. Fast Cure Cum Binder can use without any changes to the existing process. Generally it has been found that a short steam cure of 2 to 3 hours with more than 80OC on the plates leads to a very well developed tetra-basic crystal structure and helps to improve the adherence of the paste on the grid, especially if the more corrosion resistant lead alloys are used. Thereafter, the plates are curing that was implemented to speed up the free lead reduction. It has also found to be important to have a good air circulation during the final drying to ensure the reaction of the small parts of residual free lead content below 1%. By use of Fast Cure Cum Binder effect of a homogenous crystal structure with a uniform and high porosity with good penetration of the acid into the active material provides an excellent positive plate for the lead-acid battery performance. It can be observed that the conventional methods come up with a lot of small sized crystals in the range of 0.05 to 3 micron. The tetra basic cured paste was adjusted to a crystal size of 8 – 13 micron by addition of 1 %.The applied high temperature curing converted more than 98 % of the sulfuric acid into tetra basic lead sulfate. Sulfuric acid as one of the construction materials is limiting the growth of the tetra basic crystals therefore it is possible to calculate the amount of seeds needed to adjust to a certain crystal size if the paste preparation method is validated. The results from the mercury high pressure porosimetry measurement are demonstrating the big difference of the pore size diameters and also of the pore areas and the porosity values.
As expected tri basic cured pastes have great pore areas but only a small part of this area is available for the electrical performance of the battery. Most of the small pores became clogged very soon during battery operation and therefore are not supporting the cold cranking capacity. At least the charge acceptance of the battery was reduced by clogging of the pores. By use of Fast Cure Cum Binder crystal sizes are able to withstand the clogging effect over a much longer period, because the pore diameter is bigger and supports the acid exchange within the pores. Porosity values of more than 50 % are indicating improved deep discharge performances due to the good acid transport within the whole paste.
By the use Fast Cure Cum Binder Following Significant result would obtained :
- The curing & drying time can be shortended to 1 day as compared with 2 to 4 days at present.
- By use of Fast Cure Cum Binder gives controlled crystal sizes of 3 to 15µm and narrow distribution of the Tetra basic lead sulfate crystals increasing the porosity.
- It assists the supply of acid to the positive active material on increasing the porosity of the paste to enhance diffusion. The addition of CMC was found to increase the water absorption of the paste with improved mechanical strength of the paste. This is due to the dispersion stabilizing properties of CMC. Vibration test shows marked improvement. It shows improvements in mechanical strength of active materials during all process and cycle life.
- It increases the water accumulating capacity of the paste & influence the crystallization of the mass lead into stabilizing the active mass structure.
- Formation is similar to that of regular process saves 10-15 % less electrical energy.
- No activation problems with dry-charged batteries. After being filled up with acid, dry charged batteries are operational without the need for additional charging.
- The battery performance is increased and the cycling behavior is improved due to the Tetra basic lead sulfate with residual alpha-PbO avoiding deep discharge. 2
- Due to increased porosity of the active the small crystal structure with higher surface area,it maintains high ionic conductivity, Better charge acceptance of deep discharged batteries due to higher alpha-PbO 2
- Fast Cure Cum Binder can be used with different kinds of lead oxide. It gives the same results for the crystal size for ball mill oxide and Barton pot oxide.
- By the use of FAST CURE CUM BINDER Stronger paste grid adhesion is achieved & More stable structure of the positive active material is obtained ultimately Shelf life of Lead Acid batteries is increased.
Dosage : 1 Kg per 100 kg. Of oxide material.
Available Packing : 25 kg. HDPE Bag.
Storage : Store in a cool & dry place.
Tubular Positive Additive (TPA) | Download |
(For Tubular Positive Dry Blend Filling)
20-25% Extra Backup Guarantee.
Optimum Oxide Material Maximum Utilisation
The effect of addition of Tubular Positive Additive shows when the tubular electrodes were cycled; the capacity of the ground positive active material was gradually restored. Energy density of Active material & Cycle life increased as a function of the contact of Tubular positive additive. The active material on the positive plates is mainly a mixture of lead oxide and Red Oxide. In regular formulation, some of the active material will be underutilized due to non porous structure, Lack of Tetra basic lead sulfate Crystals Structure. In order to achieve a porous structure with a high surface area for the electro-chemical process, the Tubular positive plates undergo a high temperature curing process to transform the lead oxide and lead sulfate into Tetra basic lead sulfate.
There is however limitations due to the large size of the Tetra basic lead sulfate crystals. This can lead to a major disadvantage with Tetra basic lead sulfate namely a prolonged and higher electrical energy requirement for the formation of the positive plates and less suface area for the electro-chemical processes. We suggest that after dry blend filling dip Tubular Positive electrodes in 1.150 Specific gravity of H So and then cured at 85oC for 4-5 24 housrs in oven to from tetra basic Lead Sulfate crystals and alpha PbO . The tetra basic lead sulfate has sizes up 2to 3-20 micron. Tubular positive additive leads into Uniform crystal size and increase in specific surface area. The formation of a homogenous tetra-basic sulfate crystal structure in the Tubular positive plates of lead-acidbatteries is well known to offer significant electrochemical improvements in battery performance in respect of improved cycling and battery life.
By addition of Tubular Positive Additive a number of crystal seed sites are created, this leads to a large number of relatively even tetra-basic lead sulfate crystals. It has been found that only a relatively short treatment of 3 to 4 hours is required to fully develop the crystal structure in the oven. Without addition of Tubular Positive Additive in the blend Conventional method of Oven Chamber Heat Treatment Tetra basic curing process leads in the appearance of relatively large Tetra basic lead sulfate crystals. Up to now it is has not been possible to control precisely the size of the final Tetra basic lead sulfate crystals.
The uncontrolled Tetra basic crystal growth leads to a reduction in the active surface of the plage. A high specific surface area is demanded for good battery performance with respect to high current discharge capability.
The main feature of the Tubular Positive Additive is that the size of the final Tetra basic lead sulfate crystals can be controlled within the range from 4 to 15µm. Small crystals with a narrow distribution exhibit a l arger specific surface in Tubular Positive Additive allows the reduction of the curing time by use of steam. It is a generally held belief that the reduction of the residual metallic lead is only achieved within such a curing step. It is easy to prove that the free lead reduction can place during the drying phase by ensuring that there is sufficient oxygen/air available. Nevertheless the appearance of large Tetra basic lead sulfate crystals of 50 μm and above give problems during formation and,reduce the specific surgace area. It has been well tested that the addition of Tubular Positive Additive generates small and controlled sized Tetra basic lead sulfate crystals. The Tubular Positive Additive Contanis a very fine-sized and specially treated Tetra basic lead sulfate with LR Grade Calcium Sulfate. Tubular Positive Additive can use in dry blend filling with 50% Red Oxide and 50% Grey Oxide ratio. Generally it has been found that a short steam cure of 4 to 5 hours with more than 80oC on the plates leads to a very well developed tetra-basic crystal structure and helps to improve the adherence of the paste on the grid, especially if the more corrosion resistant lead alloys are used. Thereafter, the plates are curing that was implemented to speed up the free lead reduction. It has also found to be important to have a good air circulation during the final drying to ensure the reaction of the small parts of residual free lead content below 1% by use of Tubular Positive Additive effect of a homogenous crystal structure with a uniform and high porosity with good penetration of the acid into the active material provides an excellent positive plate for the lead-acid battery performance. The pore diameter is bigger and supports the acid exchange within the pores. Porosity values of more than 50 % are indicating improved deep discharge performances due to the good acid transport within the whole surface area.
About 10% Higher Initial Capacity & 6% higher average capacity is clearly differentiable between the cells containing Tubular Positive Additive & cells without Tubular Positive Additive. It can be observed immediately by testing difference of previous lots and addition of Tubular Positive additive lots. By the Use of Tubular Positive Additive stable Positive active mass structure Obtained. The battery performance is increased and the cycling behavior is improved due to the Tetra basic lead sulfate with residual alpha-PbO2 avoiding deep discharge. The most stable capacity PAM structure develops and it increases the rate of Lead Sulfate nucleation during discharge and modifies the beta-PbO and lead sulfate structure. Tubular Positive 2 additive increases the voltage at all concentrations and discharge rates, and the effect was more pronounced at higher currents and lower temperatures. Ultimately Shelf life of Tubular Lead Acid batteries is increased.
Dosage : 2 kg per 100 kg. of oxide Dry Blend mixture.
Available Packing : 25 Kg HDPA Bag.
Storage : Store in a cool & dry place.
Polypropylene Fibers (3mm) | Download |
PP Fibers : (For Automotive, VRLA Positive Plates)
SSRL supplies a range of percision cut fibers cut from 0.5 mm to 4mm for mechanical reinforcement to the lead oxide paste on the grid of battery plate, thereby reducing oxide shredding and preventing premature failure of the battery. SSRLPP fibers improves the life of battery, thus making the battery last longer. The fiber is resistant to acids, alkalis, most organic solvents, oxidizaing agents and heat. Precession cutting and proprietary finish processing enables ultra effective dispersion under all conditions without agglomeration and clumping.
Product Image :
PRODUCT SPECIFICATION : SSRL PP 03mm
Dosage : 1 gm. per Kg. Oxide Material.
Packing : : 20 Kg. HDPE Bag.
Storage : Store in a Cool and Dry Place.
PTFE Solution | Download |
PAM EFFICIENCY ACITIVATOR : (For Automotive, Tubular , VRLA Positive Plate)
The Lead Acid Battery having many applications, these inculde high specific power and power density, high volumetric energy density & specific energy (energy per unit weight). There is growing demand for the development of lead-acid batteries because of reduced cost & recycling. The need for electrical energy storage has increased progressively up until present time & shows no sign of abating, The need for improved energy storage devices to handle the increased load & capacity has followed the incremental usage of electrical power devices in automobiles. Larger engines, higher crank speed, and increasing customer expectation have motivated the industry to provide higher specific energy and longer service life.
The theoretical specific energy of the lead acid battery is 218Wh kg. -1 for 100 wt.% sulfuric acid. This value is based on a cell voltage of 2.606V for 100% sulfuric acid going to 100% water on battery discharge. If it is assumed that 40% sulfuric acid is used and the weight of water is inculded, then the theoretical specific energy drops to 123Wh kg-1. The specific energy actually achieved depends on the discharge rate but is around 20% of the theoretical capacity. The major reason for the low specific energy is that much of the active material in both the positive and negative plates is not discharged. Thus, non reacting active material provides structure and conductivity to the plates, but do not contribute to the cell reaction.
Lead Acid Battery Technology proved for above applications low cost, high power capability, excellent low temperature performance, safety under all circumstances & longer shelf life. Today it is a need for improvements in active materials utilization. The actual specific energy of about 30Wh Kg-1 for most commercial models to the theoretical maximum specific energy of 166 Whkg-1, required. Even though to maintain a good life, the battery must be shallow discharged so that the discharge required for the engine start must be less that 5% of rated Capacity. Pam Efficiency Activator is incorporated in the composite electrochemically active material carried on the lead plates of a lead-acid storage battery. In the case of the positive electrodes, the result is to lengthen the service life of more than 20%.
Product Image :

Pam Efficiency Activator offers a battery plate that virtually eliminates flaking and shedding of positive plate active materials i.e. the main reason for battery failure. The Pam Efficiency Activator forms fibers, and these fibers create a complex matrix that traps and binds the active material together. The result of shedding and flaking are virtually eliminated, the lead paste remains cohesive, and contact with the positive grid remains in place for a longer period.
Pam Efficiency Activator improves the electrochemically active composite masses used on the grid metal alloy, the density of the composite mass is reduced and its water-holding properties increased. During the curing process, the paste within the grid becomes hard. As the oxidation of Pb proceeds and lead basic sulfate crystals grow, the water content thereof partially vaporizes, subsequently giving rise to cementation phenomena which bonds the oxide particles together, resulting in the grid hardening. During the charge and discharge cycle, a paste type positive electrode plate repeatedly expands and shrinks and eventually swells by producing fine particles so that the active material is dropped out from the grid, thus resulting in shortening of the cycle life. Pam Efficiency Activator forms a mesh-type network involving active material particles, so as to avoid dropping out.
Pam Efficiency Activator shows remarkable incomplete active material utilization improvement as follows : Pam Efficiency Activator working on paste density & chemical changes that improve ionic conductivity or electronic conductivity in the positive active material.
Utilization (%) – Qout/Qtheo
Specific capacity (Ah/kg) - Qout/mtot
Specific capacity (Wh/kg) - Eout/mtot
Positive active material – PbO2
Pam Efficiency Activator has shown that the ampere-hour capacity of lead-acid batteries increases, i.e. the efficiency of the electrically formed active material raises, with an increase in the amount of sulfuric acid and/or water used in the preparation of the paste. This increases the porosity and thus the surface area of the positive electrode active mass.
How the positive active material works in a Lead Acid Batteries.
Pb(IV)O2 + SO42- + 4H+ + 2e- = Pb(II)SO4 + 2H2O
Ion conductivity
- Sulfuric acid is a reactant
- Reaction limited by diffusion at fast discharge
Electrical conductivity
- PbO2 is a conductor, PbSO4 is not
- Reaction limited by paste conductivity at slow discharge
At fast discharge rates, the hydrogen sulfate is consumed faster than it can diffuse into the plate, limiting the overall PAM Efficiency Activator increase in utilization at a fast discharge rate. Pam Efficiency Activator significantly protects grids from corrosion & increases mechanical strength of the plate.
PAM efficiency Activator increases specific energy and porosity of the plates. If the porosity of the positive active material is increased, the diffusion of electrolyte into the plate is enhanced and thus more of the active material will react, which again increases the specific energy. The magnitude of both these effect depends on both the discharge rate & the battery design.
The maximum increase in utilization is reached with addition of PAM Efficiency Activator. The positive grid is subject to corrosion the rate of this debiliating process is influenced by grid composition & microstructure, Plate potential, electrolyte composition, temperature.
The corrosion is generally more electrically resistive than the grid & thus diminishes the output of the battery in extreme cases, corrosion results in disintegration of the grid & collapse of the plate.
The use of lead antimony alloy enhances the creep strength of the positive grid & thus retards growth in the plane of the plate. For the flat design of positive plates, expansion normal to the plate can be moderated by applying a compressive force to the plate group. PAM Efficiency Activator provides binding protective shell to paste while charge and discharge mechanism, thus it provides long lasting reinforcement to the paste on the battery plate. By use of this positive plates have long cycle life and high coefficient of utilization.
High charge retention at long storage time in wet condition.
Lead/acid batteries are produced in sizes from less than 1Ah to 3000Ah for a wide variety of portable, Industrial and Automotive applications. Designs include pasted and tubular electrodes. In addition to the traditional designs which are flooded with sulfuric acid, newer “valve-regulated” designs have the acid immobilized in a silica gel or absorbed in a porous AGM separator.
Development is ongoing worldwide to increase the specific power, energy and deep discharge cycle life of lead acid battery electrodes. Therefore, Pam Efficiency Activator provides all types of electrode plates for the lead-acid battery having a wide range of applications and great value in industry.
Dosage : 1000 ML per 100 kg of oxide paste material.
Packing : 50 Lit Carbo drum.
Storage : Store in a cool & dry place.
IBR Additive | Download |
Instant Backup Revival Additive (For Revival of Backup in Lead Acid Batteries)
Environment Friendly IBR Additive for old Lead Acid Batteries IBR Additive Restores Sulfated Lead Acid Batteries. Sulfation is a Common natural process occurs during discharging. When the battery is deeply discharged or left on open circuit in the discharged condition, abnormal condition will leads into hard sulfation. IBR ADDITIVE, which is used to dissolve sulfate deposits from lead acid batteries.
Effective product for old Lead acid batteries Instant Backup Revival Additive, Which is developed for troubleshooting and old battery revival purpose only. IBR Additive is developed by innovative advanced technology. It is a very efficient lead acid battery additive for automotive and industrial battery, especially Tubular, Traction batteries and other deep cycle industrial batteries.
The main functions of IBR Additive are as follows :
Remove Sulphation : Sulphation is the main cause of battery failure. IBR ADDITIVE is able to eliminate "irreversible" sulphated compound on battery plates and prevent sulphaton.
Figure (A) shows : The new Active material plate has a spongy shape. This shape has a large surface area for the chemical change. Therefore, the new active material plate can make the chemical change smoothly.
- Charging efficiency is high.
- The battery can save the electricity fully.
- Discharging power is high.
Figure (B) shows : The crystallized "Sulfation" covers over the active material plate. Sulfation means the lead sulfate (PbSO4), this substance makes the battery life shorter & does not carry the electricity. The surface of the element plate has a high resistance by this condition, therefore, the old active material plate cannot make the chemical change smoothly it leads into
- Charging efficiessncy is low.
- The battey cannot save the electricity fully.
- Discharging power is low.
According to studies International Battery Council, 60 to 70% batteries are replaced prematurely due to the sulfation that occurs on lead plates. Sulfation is an electrochemical reaction that occurs when a battery is discharged. During normal use or in storage, a battery's sulfuric acid is active between the plates. This reaction creates energy in the form of electric current. It also transforms the acids chemical composition i.e. in contact with the lead plates, forming a solid residue(Lead Sulfate). Finally, the loss of sulfuric acid reduces the electrolyte's sulfuric acid in liquid state becomes solid and the electrolytes specific gravity reading drops. However, after charging the battery, the solid crystal residue will turn into liquid again but doesn't allow the lead sulfate to turn totally into liquid (at 100%). Some residue will remain fixed on the plates or fall down to the bottom of the battery.
Sulfation reduces the electrolyte concentration and, as a consequence the cell voltage is also reduced. As sulfation increases the internal resistance increases & the extra heat generates which produces a mark rise in electrolyte temperature. These higher temperatures then further accelerate the dry out process often culmination in the failure of battery. This Continual Sulfate Accumulation Accelerates The Weakening Process And Finally “suffocates” The Battery.
Symptoms of Sulfated Batteries :
- A General Lack of Capacity(Deliverable Power)
- An Increase in Battery temperature while charging & discharging
- A strong Odour of Hydrogen Gas while charging
- An excessive use of water
- A rapid rise in battery voltage while charging
- A sudden drop of Battery voltage while discharging
Under certain conditions of over discharge, the amount of dissolved lead sulfate may be such that, on recharging, the reduced lead will form metallic bridges between the plates (dendrites).
Sulfation makes worse the battery condition
- Sulfation makes the battery power down and worse the condition. In case of the vehicle
- The starter motor doesn't run well.
- The power windows move slowly.
- The head light beam gets dark.
- The battery fluid tends to evaporate in the short term.
In case of the electric vehicle
- The running time is short.
- The motor power decreases.
- The battery tends to heat up.
- Although the lead acid battery is charged, it does not work well.
If the Sulfation problem is resolved by advanced SSRL technology, we can prolong the battery life and improve battery reliability.
Starter battery can revive and prolong the lifetime
- Revive (Go up the acid density) the starter battery and extend the life
- Resolve the Sulfation even the used lead-acid battery
- The new battery can keep fresh condition
- Stable battery power under the high load
- Easy to start engine under the low to high temperature
- Brighten the headlights beam
- Stable battery power even air conditioner on
- Electric car accessories work stably
- Enhance the performance of the car audio & AV system
- Prevent the battery flat on the drive scene
- Prevent the battery aging for the idle stop cars
- Prevent the battery aging for the auto on headlights cars and motorcycles
- Easy battery maintenance - Evaporation of the battery fluid goes down
- The load of alternator goes down
Increase Capacity : By removing sulphation, IBR ADDITIVE can repair and improve conductivity of battery plates. The lost capacity can be recovered effectively by charging.
Extend Battery Life : IBR Additive extends battery life by removing and preventing sulphation build up.
Higher Power Output : By removing sulphation and improving conductivity, battery with IBR ADDITIVE will have higher power output or higher cranking current.
IBR Cannot Work in Below Conditions of Lead Acid Batteries :
- Not all lead-acid batteries can revive and extend the battery life. Of course, SSRL technology cannot work well on the physical damaged batteries.
- The Active material of plates are damaged under the lack of battery electrolyte.
- The lead-acid battery get a shock physically and Active material of plates and separators are damaged i.e shredding of active material.
- The Active material of plates and separators have physical problems such as corrosion, collapse and short-circuiting.
- Breaking of intercell welding etc.
After Normal Charging If one or more cells are giving exceptionally high bubbles or gassing then it is possible that such cells have heavy shedding of active materials, separator puncture, mechanical short, or sludge formation at the bottom area. Such cells will not give any improvement in the gravity and will remain same as it was before battery was put to charge. Such batteries are discarded.
If you find that there is an improvement in battery volts and gravity in each cell, such batteries is selected for IBR treatment.
After addition of IBR mix it well with electrolyte using hydrometer. Reddish yellow color will be there in electrolyte. Leave the battery in idle condition till the electrolyte becomes colorless. This may take 12 to 24 hours. The less sulfated healthy cells will have faster discoloration and more sulfated cells will have slower discoloration. If the cells are mechanically damaged then there will not be discoloration.
Chargeing A Battery On Pulse Rejevenator :
After the discoloration of electrolyte has been completed, carry out normal battery charging using different amps for different ah capacity batteries. Continue to charge the battery on Pulse Rejuvenator till full gravity is observed in each cell and the battery volts reached above 15.5V for a 12V battery and For traction battery it should be 2.6volts per cell..Fully charged battery is then removed from Pulse Rejuvenator machine and allowed to settle to room temperature, note the volts and gravity in each cell. You will find that the battery is absolutely Revived.
VRLA Gel Battery Rejuvenation With IBR
For VRLA GEL batteries Revival,First Weigh the Old Battery and Compare Weight Loss. If you have find a weight loss then remove plastic strip on the top of the battery case. And add IBR 5 ml per liter of electrolyte volume dosage in each and every cell. After addition of IBR add distilled water up to plate height in each cell & mix electrolyte then leave the battery for 12 hours in idle condition during this time IBR will go to battery plates. After 12- hours carry out Pulse Revenunation ChargingProcdure for next 12 hours.
Results will be – All Cells Voltage / Gravity / Back Up – shows Satisfactory Magical Performance.
IMP Note
- IBR additive fully tested in SSRL laboratory & taken more practical trials in global market since last 10 years.
- No harmful effects observed in all types of lead acid batteries while treatments & after treatments.
Dosage of IBR Additive : IBR to be used in old lead acid batteries 5 ml per lit. of electrolyte volume in all cells.
Packing : 1 kg. Bottle packed in 16 kg. DFC Box.
Storage : Store in a Cool and Dry Place.
Dross Free Alloy Additive | Download |
Dross Free Alloy Additive : (To be use in Lead Melting Pot of Lead Alloy Antimony & Selenium)
DFA Additive is mixture which can separate metal liquid with oxidation dross. It is used for reducing lead dross and removing dross during lead alloy making and grid making. It can collect the valuable metal and reuse it again to improve the metal usage, saving the cost.
Principle :
- It can change the surface and interface tension and reduce the combining power between metal liquid and dross, to make the metal liquid separate with dross, improving the lead usage.
- It can create a lot of bubble after putting the chemical into the liquid which has the function for lead liquid mixing. It can make the dross stirring totally in the metal liquid to drive up to the surface which can be taken out easily and make the pot clean.
- The chemical has the function for dross adsorption and melting to reduce the dross, and make the metal using fully.
Feature :
- This chemical can separator the valuable metal in the metal dross, reducing the valuable metal taken away because of removing dross. It improves the usage of metal upto 4-5%, saving the production cost.
- There is less smoke coming during using lead dross elimination Chemical, no poisoning in air and Inhalation will leads into all peoples safety, reducing the equipment corrosion because of corrosion air during lead melting. And it makes the lead separate with dross totally.
- It is easy to use, safe and convenient. If the chemical is wet and agglomeration, please make it baking properly to remove the moisture.
- The chemical can keep the dross temperature for a long time, so the valuable metal can flow out totally from the dross and reuse again.
Usage :
The chemical should be put in the lead melting pot uniformly covering the surface of lead liquid, stirring slowing to make the chemical mixing with lead liquid totally. If the lead liquid has too much dross, it can use a spoon which has chemical inside to press the chemical into the lead liquid, and moving slowly inside. After the dross floating on the surface of liquid naturally, and the temperature reach one point. This time the dross is loose, take out the dross from pot. After all dross is taken out, stirring the dross totally in order to the lead flowing out and reuse again.
There are two models of lead dross elimination chemical.
Dosage : 250 Gm .Per Tonne Lead Alloy Material.
Packing Size : 25Kg.HDPE Bag.
Storage : Store in a Cool and Dry Place.
Battery Terminal Protector | Download |
We have our Best Product to Protect Battery Terminal and Internal Resistance, Manufacturers should put with each and every final Packaging of Lead Acid Battery.
Product Details are as below.
- Highly Pure Grade Synthetic Jelly with Special Anti corrosive Additives
- Effective against Corrosion on Battery Terminals.100% Protection of Battery Terminals.
- Smoothens Current flow while charge & Discharge Cycles.
- Cheaper and More Effective.
Product Image :

How to Use :
For Better Results Wash the Battery Terminals by water and dry the terminals and apply Battery Terminal Protector on both positive and negative terminals thoroughly.
Specifications :
Description : White Translucent mass retaining the character slightly fluorescent by day light. Odorless & Tasteless.
Melting Point : 50OC
Sulphated Ash : Nill
Packing No. Qty : 5gm Pouch / 1600 No.s of 01 DFC Box
Contains : Pure Petroleum Jelly USP & Anti Corrosive Additives.
Sodium Sulphate LR Grade Powder | Download |
We manufacture sodium sulphate LR Grade that is a vital compound of sodium. Sodium sulfate is the sodium salt of sulfuric acid. When anhydrous, it is a white crystalline solid of chemical formula Na2SO4
Specification : Sodium Sulfate LR Grade Anhydrous - Na2SO4
| Test | Result |
| Assay | 99.0% Na2SO4 |
| pH of a 5% solution | 6.2-9.2 at 25C |
| Insoluble matter | 0.01% |
| Loss on ignition | 0.50% |
| Chloride (Cl) | 10ppm |
| Nitrogen compounds (as N) | 5 ppm |
| Phosphate (Po4) | 0.01% |
| Heavy metals (as Pb) | 5 ppm |
| Iron (Fe) | 10 ppm |
| Calcium (Ca) | 0.01% |
| Magnesium (Mg) | 0.01% |
| Potassium (K) | 0.01% |
Standard Packing : 25 kg. HDPE Bags.
Storage : Store in a cool and Dry Place.
Warning : Do not use commercial Grade Sodium Sulfate Powder, it contains more Chloride and Iron impurities which is harmful for Lead Acid Batteries.
Batt. Wash Neutral Soap - SSBW | Download |
Specifications :
We are one of the providers of a Battery Washing Neutral Soap Solution For Lead Acid Battery Industry , to Clean the batteries after formation or after charging. Batt Wash Concentrate Neutral Soap Solution that are designed in compliance with international quality standards. The battery wash neutral soap solution product is precisely manufactured by making use of high grade basic material. Moreover, these products are availed at industry affordable prices from us to our all lead acid battery manufacturers .
Features :
- v Accurate composition
- v Fast & easy wipe off
- v No reaction with inside acid of lead acid battery
- v Quality assured
- v Green in color
- v Fragrance - Jasmine
Direction For Use : Add 1 Part Conc. to 50 parts of water in a clean bucket ( About 70 ml in a 4 lit water) scrub & rinse the battery thoroughly to remove dirt & acid. Wash the battery using a clean , soft sponge , towel or wash mitt. Rinse thoroughly with water & dry using clean, soft or terry cloth towel.
Precautions : Store the material in clean dry place at room temperature storing in metal container is not recommended. During Longer Storage Process Shake Before Use if required.
Standard Packing : 60 kg. Carbo plastic durum.
Storage : Store in a cool and Dry Place.
Modacrylic Fiber (Goonvean) | Download |
PRODUCT DATA SHEET |
|
PRODUCT DETAILS |
|
PRODUCT CODE |
AC3/30 |
FIBRE TYPE |
Acrylic Fibre |
FIBRE FORMAT |
Precision Cut (Rotary) |
APPEARANCE & LUSTRE |
Yellow/Ecru fibres |
FIBRE LENGTH |
3.0mm ±0.3mm (>98%) |
FIBRE DIAMETER & DECITEX |
10-19μm/1.1-3.3dtex ±10% (>95%) |
TYPICAL PACKAGING |
20kg/carton, 720kg/pallet, 36cartons/pallet |
RELEVANT SDS |
Acrylic |
CHARACTERISTICS |
|
DISPERSION IN WATER |
Homogeneous with stirring |
COMMERCIAL MOISTURE REGAIN (%) |
1.5 |
TYPICAL MOISTURE CONTENT (%) |
1.0-2.0 |
COMMERICAL MOISTURE ALLOWANCE (%) |
<2.0 |
MELTING POINT (°C) |
Non-melting (Decomposes >280 (TGA Analysis)) |
FLAMMABILITY |
Supports combustion, forms beads |
DENSITY (g/cm3) |
1.17-1.18 |
ABSORBENCY |
Medium |
DRY TENACITY (cN/dtex) |
4.5-8.0 |
DRY ELONGATION AT BREAK (%) |
10-40 |
CHEMICAL RESISTANCE |
Good against: Acids, Most Organic Solvents, Oxidising Agents, Sunlight, Alkalis |
BIODEGRADABILITY |
Non-biodegradable |
ADDITIONAL INFORMATION |
None |
© Goonvean Fibres Ltd - Reg No. 04735337. Registered Office: St Stephen, St Austell, Cornwall, United Kingdom, PL26 7QF. Registered in England CONDITIONS: All information is supplied in good faith as being typical of the product but no warranty is given or implied. Information contained in this Product Data Sheet (PDS) supersedes all previous such information and is based on the Company’s knowledge at the date of preparation. Ref: PDS Issue: 2/12
Tetra basic lead sulphate | Download |
Tetra Power (TBLS) required for positive active material paste where tetra basic seeding fine crystals are required , to generate more % of TBLS in active mass structure. Its distribution in active material is more uniform and crystals are evenly distributed. The curing profile can be done at lower or Higher temperature with saving in energy, thus Curing and Drying time shortened.
Product Image :

Carboxy Methyl Cellulose (CMC) | |
Not Applicable
Cork Powder | Download |
Not Applicable
Sodium Lauryl Sulphate (SLS) | Download |
Not Applicable
Stearic Acid |
Not Applicable